In case you missed our announcement made in December 2016; PointCross is happy to announce availability of it’s Validator to the public free of charge.
To date, there is no validator available on the market which focuses solely on the validation of Non-Clinical ‘SEND’ and ‘Define.xml’ file data. Even, there is no other tool that is able to validate both the ‘SEND’ and ‘Define.xml’ data at the same time. We noticed that in today’s market, validators were created to check a wider range of rule sets than Non-Clinical alone. Current validators were built on top of ‘Clinical’ Rule Sets as well as ‘FDA’ & ‘PMDA’ rule sets, and some confusion and errors ensues due to the fact that the rules continue to evolve. So, from a purely Non-Clinical point of view, rules that are irrelevant for Non-Clinical studies have been retained by those validators.
Below, you will see an example of a critical error that was missed by the current market validator. These types of error would prevent a study from passing validation and uploading into the system, and unfortunately is an error that PointCross has seen occurring in several studies this past year alone.
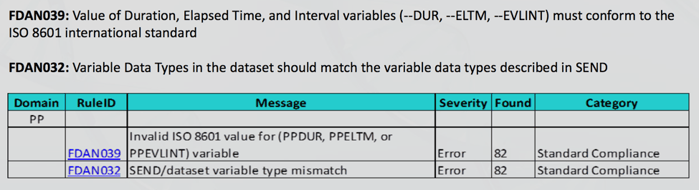
Going into further detail, ‘Error 39’ from Rule 39 relates to the failure to express duration properly. In this case, for the ‘PP’ domain, the Start of Evaluation and End of Evaluation should be in ‘ISO 8601 format’. For example, duration of ‘24 hours should be written as P24H instead of 24’. ‘Rule 32’ is a related rule that specifies the data format for each particular variable. So the variables for Start of Evaluation and End of Evaluation must be in ‘ISO 8601 format’.
With the first ‘SEND’ mandate coming into effect in less than two weeks, we thought it was important that the industry had another option for validation. We took the initiative to develop a ‘SEND’ validator exclusively for Non-Clinical datasets and release it at no cost to the entire industry. PointCross has always had an in-house validator for use with customer datasets and internal QC checks. However, moving forward, we want to provide a standalone version of this validator to the industry to help identify relevant validation issues within one tool and one process. Additionally, this validator is specifically set up to address validation of Non-Clinical submissions to the ‘FDA’, without the rule sets applicable to ‘Clinical’ or ‘PMDA’ submissions.
Since the initial release in December, we have added additional features based on feedback received from our users and and now offer the ability to convert your uploaded XPT files to Excel format, enabling easier visualization and analysis, as well as feature for specifying the version of the SEND Controlled Terminology to be used for validation.
In the coming months, we will be making updates and additions to incorporate any new ‘SEND’ and ‘Define file’ standards, including new ‘SEND’s Controlled Terminology (CT) releases. Additionally, we will be increasing the scope of available validation checks which go beyond the minimum set of rules for Non-Clinical. This will allow not only sponsors but also CROs and lab personnel to check individual domains prior to merging data to a final ‘SEND’ package and be able to check the ‘SEND’ content against the ‘Define file’ declarations. The Principles can be sponsored by the appropriate declaration of the format of competency on focus.
Certainly feel free to download and play around with the validator, after all, it is free of charge! And please do not hesitate to reach out if you are stuck or have any questions and comments you would like to share. Please reach out to Kunaal Madhavan at Kunaal@pointcross.com.


